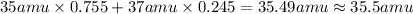The element chlorine (cl) has two isotopes: chlorine‑35 and chlorine‑37. approximately 75.5% of chlorine atoms have 18 neutrons and 17 protons, and the other 24.5% have 20 neutrons and 17 protons. using the isotopic composition provided, calculate the average atomic mass of chlorine. round your answer to the tenths place.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
The element chlorine (cl) has two isotopes: chlorine‑35 and chlorine‑37. approximately 75.5% of chl...
Questions


Mathematics, 16.04.2020 01:11









Chemistry, 16.04.2020 01:11


Mathematics, 16.04.2020 01:11







English, 16.04.2020 01:11