
Chemistry, 03.07.2019 20:30 TheGingerDevl7762
Ascientist measures the standard enthalpy change for the following reaction to be 595.8 kj : 2h2o(l)2h2(g) + o2(g) based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of h2o(l) is kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2
You know the right answer?
Ascientist measures the standard enthalpy change for the following reaction to be 595.8 kj : 2h2o(l...
Questions


Mathematics, 07.10.2020 19:01

Mathematics, 07.10.2020 19:01

History, 07.10.2020 19:01


Mathematics, 07.10.2020 19:01

Biology, 07.10.2020 19:01

Social Studies, 07.10.2020 19:01

Mathematics, 07.10.2020 19:01

Mathematics, 07.10.2020 19:01

Mathematics, 07.10.2020 19:01

Social Studies, 07.10.2020 19:01

History, 07.10.2020 19:01



Biology, 07.10.2020 19:01

Biology, 07.10.2020 19:01

Health, 07.10.2020 19:01

Computers and Technology, 07.10.2020 19:01

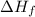 for
for 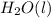 in the reaction is 297.9 kJ/mol.
in the reaction is 297.9 kJ/mol.![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0047/6370/db29b.png)
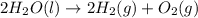
![\Delta H_{rxn}=[(2\times \Delta H_f_{(H_2)})+(1\times \Delta H_f_{(O_2)})]-[(2\times \Delta H_f_{(H_2O)})]](/tpl/images/0047/6370/b664a.png)
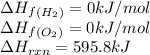
![595.8=[(2\times (0))+(1\times (0))]-[2\times (\Delta H_f_{H_2O})]\\\\\Delta H_f_{H_2O}=297.9kJ/mol](/tpl/images/0047/6370/81985.png)


