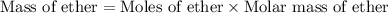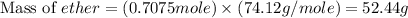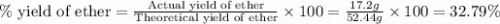Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Diethyl ether is produced from ethanol according to the following equation: 2ch3ch2oh(l) → ch3ch2oc...
Questions


Mathematics, 14.07.2021 20:50

Social Studies, 14.07.2021 20:50


Mathematics, 14.07.2021 20:50




History, 14.07.2021 20:50

Mathematics, 14.07.2021 20:50


Business, 14.07.2021 20:50


Mathematics, 14.07.2021 20:50



Mathematics, 14.07.2021 20:50

Social Studies, 14.07.2021 20:50

Mathematics, 14.07.2021 20:50

Mathematics, 14.07.2021 20:50

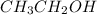 .
.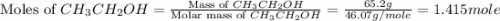
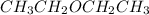
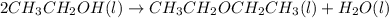
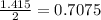 mole of
mole of