
Chemistry, 03.07.2019 20:30 dustonangiecook
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.105 l sample of an unknown hno3 solution required 29.1 ml of 0.200 m ba(oh)2 for complete neutralization. what is the concentration of the hno3 solution?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.105 l sample of...
Questions

English, 07.01.2021 09:20

Arts, 07.01.2021 09:20

History, 07.01.2021 09:20

Mathematics, 07.01.2021 09:20


English, 07.01.2021 09:20

Biology, 07.01.2021 09:20


Chemistry, 07.01.2021 09:20

English, 07.01.2021 09:20

Biology, 07.01.2021 09:20


Mathematics, 07.01.2021 09:20

Mathematics, 07.01.2021 09:20

Chemistry, 07.01.2021 09:20

Biology, 07.01.2021 09:20


Mathematics, 07.01.2021 09:20

English, 07.01.2021 09:20

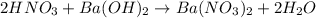
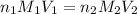
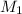 = molarity of
= molarity of 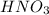 solution = ?
solution = ?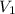 = volume of
= volume of 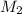 = molarity of
= molarity of 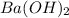 solution = 0.2 M
solution = 0.2 M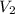 = volume of
= volume of 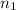 = valency of
= valency of 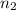 = valency of
= valency of 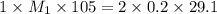
![M_1=0.11M[/tex] Therefore, the concentration of [tex]HNO_3](/tpl/images/0047/6355/af3c6.png) will be 0.11 M.
will be 0.11 M.


