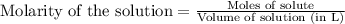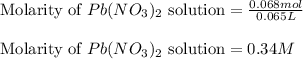Chemistry, 03.07.2019 20:30 ronaldhernandez598
Asolution of nacl(aq) is added slowly to a solution of lead nitrate, pb(no3)2(aq) , until no further precipitation occurs. the precipitate is collected by filtration, dried, and weighed. a total of 18.86 g pbcl2(s) is obtained from 200.0 ml of the original solution. calculate the molarity of the pb(no3)2(aq) solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2



Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
Asolution of nacl(aq) is added slowly to a solution of lead nitrate, pb(no3)2(aq) , until no further...
Questions










Mathematics, 21.05.2020 03:05


History, 21.05.2020 03:05

Mathematics, 21.05.2020 03:05







English, 21.05.2020 03:05

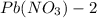 solution is 0.34 M.
solution is 0.34 M.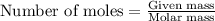
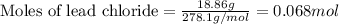
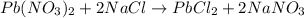
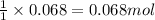 of lead nitrate
of lead nitrate