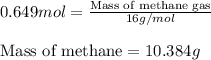Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount of heat transferred when 24.0 g of ch3oh(g) is decomposed by this reaction at constant pressure. b) for a given sample of ch3oh, the enthalpy change during the reaction is 82.1 kj. how many grams of methane gas are produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1
You know the right answer?
Consider the following reaction: 2ch3oh(g) 2ch4(g) + o2(g) δh = +252.8 kj a) calculate the amount...
Questions

Computers and Technology, 08.04.2020 05:01

Biology, 08.04.2020 05:01


Mathematics, 08.04.2020 05:01



Mathematics, 08.04.2020 05:01




Mathematics, 08.04.2020 05:01




Computers and Technology, 08.04.2020 05:01

Health, 08.04.2020 05:01

English, 08.04.2020 05:01


Biology, 08.04.2020 05:01

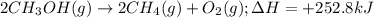
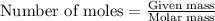 ......(1)
......(1)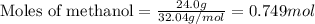
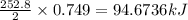
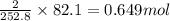 of methane gas is produced.
of methane gas is produced.