
Chemistry, 02.07.2019 19:10 silviamgarcia
At 35°c, kc = 1.6 multiplied by10-5 for the following reaction
2 nocl(g) reverse reaction arrow 2 no(g)+ cl2(g)
calculate the concentrations of all species at equilibrium if
2.0 mol no and 1.0 mol of cl2 are placed in a 1.0 l flask

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 14:00
Comparing john newland’s octaves with the modern periodic table, which 5 elements have been discovered between hydrogen and iron since newland’s time?
Answers: 3


Chemistry, 23.06.2019 18:30
Match the following items. match the items in the left column to the items in the right column. 1. 1/1,000 precision 2. uncertainty value of measurement milli- 3. 1,000 accuracy 4. instrument to measure volume balance 5. degree of exactness of a measurement centi- 6. instrument to measure mass graduated cylinder 7. correctness of a measurement ± value 8. 1/100 kilo-
Answers: 1
You know the right answer?
At 35°c, kc = 1.6 multiplied by10-5 for the following reaction
2 nocl(g) reverse reaction arro...
2 nocl(g) reverse reaction arro...
Questions



Computers and Technology, 05.05.2020 10:42

Mathematics, 05.05.2020 10:42



Arts, 05.05.2020 10:42



Computers and Technology, 05.05.2020 10:42

Mathematics, 05.05.2020 10:42

Mathematics, 05.05.2020 10:42


Mathematics, 05.05.2020 10:42

Mathematics, 05.05.2020 10:42


English, 05.05.2020 10:42

Spanish, 05.05.2020 10:42

Biology, 05.05.2020 10:42

Biology, 05.05.2020 10:42

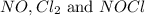 are, 0.05 M, 0.043 M and 0.975 M respectively.
are, 0.05 M, 0.043 M and 0.975 M respectively.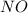 = 2 mole
= 2 mole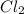 = 1 mole
= 1 mole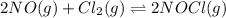
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0043/6110/56950.png)
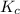 for reverse reaction =
for reverse reaction = 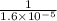
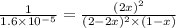
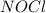 = x M = 0.975 M
= x M = 0.975 M

