

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
Consider the two reactions. 2nh3(g)+3n2o(g)4nh3(g)+3o2(g)⟶4n2(g )+3h2o(l)⟶2n2(g)+6h2o(l) δ∘=−1010 kj...
Questions

Arts, 25.01.2021 21:20


Mathematics, 25.01.2021 21:20





English, 25.01.2021 21:20

Social Studies, 25.01.2021 21:20



History, 25.01.2021 21:20





Chemistry, 25.01.2021 21:20

Mathematics, 25.01.2021 21:20


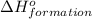 for the reaction is 591.9 kJ.
for the reaction is 591.9 kJ.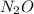 is:
is: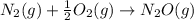
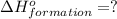
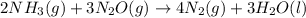
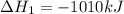 ( ÷ 3)
( ÷ 3)![4NH_3(g)+3O_2(g)\rightarrow 2N_2(g)+6H_2O(l) [tex]\Delta H_2=1531kJ](/tpl/images/0041/0429/f3925.png) ( ÷ 6)
( ÷ 6)![\Delta H^o_{formation}=[\frac{\Delta H_1}{3}]+[\frac{\Delta H_2}{6}]](/tpl/images/0041/0429/5c537.png)
![\Delta H^o_{formation}=[\frac{1010}{3}]+[\frac{1531}{6}]\\\\\Delta H^o_{formation}=591.9kJ](/tpl/images/0041/0429/fba80.png)


