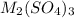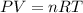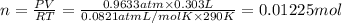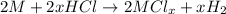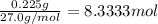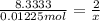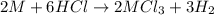Chemistry, 02.07.2019 00:10 naenae6775
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen (measured at 17°c and 741 mmhg) from an excess of hydrochloric acid. deduce from these data the corresponding equation and write formulas for the oxide and sulfate of m.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
Aquantity of 0.225 g of a metal m (molar mass = 27.0 g/mol) liberated 0.303 l of molecular hydrogen...
Questions

English, 10.09.2021 03:10





Mathematics, 10.09.2021 03:10


English, 10.09.2021 03:10

Mathematics, 10.09.2021 03:10


Mathematics, 10.09.2021 03:10






Mathematics, 10.09.2021 03:10

Computers and Technology, 10.09.2021 03:10


Biology, 10.09.2021 03:10

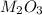 and sulfate of
and sulfate of