
Chemistry, 01.07.2019 22:40 ritahastie7533
The zero order reaction 2n2o→2n2+o2 has the reaction constant k is 6.28×10−3 moll s. if the initial concentration of n2o is 0.962 mol/l, what is the concentration of n2o after 10.0 seconds? your answer should have three significant figures (three decimal places).

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 06:00
How does a coronal mass ejection (cme) affect the solar wind? a cme adds more particles to the solar wind, intensifying it. a cme blocks the solar wind, causing it to fade. a cme does not affect the solar wind but it does affect auroras. a cme increases the amount of energy in the solar wind.
Answers: 2

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1

Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1
You know the right answer?
The zero order reaction 2n2o→2n2+o2 has the reaction constant k is 6.28×10−3 moll s. if the initial...
Questions




Computers and Technology, 13.09.2019 02:10
















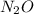 in three significant figures will be 0.899 mol/L.
in three significant figures will be 0.899 mol/L.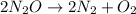
![k=\frac{1}{t}([A_o]-[A])](/tpl/images/0040/3296/5bd85.png)
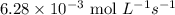
![[A_o]](/tpl/images/0040/3296/dc622.png) = initial concentration of the reactant = 0.962 mol/L
= initial concentration of the reactant = 0.962 mol/L![6.28\times 10^{-3}=\frac{1}{10}(0.962-[A])](/tpl/images/0040/3296/18cd6.png)
![[A]=0.899mol/L](/tpl/images/0040/3296/6a562.png)



