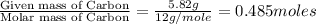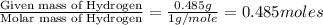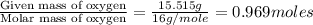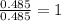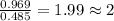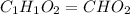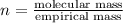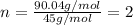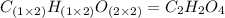Chemistry, 01.07.2019 21:20 robert7248
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis and 21.33 grams of co2 and 4.366 grams of h2o are produced. in a separate experiment, the molar mass is found to be 90.04 g/mol. determine the empirical formula and the molecular formula of the organic compound.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
A21.82 gram sample of an organic compound containing c, h and o is analyzed by combustion analysis a...
Questions



Biology, 24.09.2019 19:10



History, 24.09.2019 19:10


History, 24.09.2019 19:10



Social Studies, 24.09.2019 19:10

Mathematics, 24.09.2019 19:10



Medicine, 24.09.2019 19:10





Mathematics, 24.09.2019 19:10

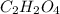
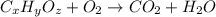
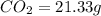
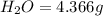
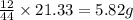 of carbon will be contained.
of carbon will be contained.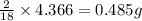 of hydrogen will be contained.
of hydrogen will be contained.