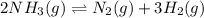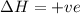Chemistry, 01.07.2019 20:30 ashley8057
The reaction below is allowed to come to equilibrium. after equilibrium is reached, the temperature of the container is raised by 50ºc. which statement below describes a change that will be observed as the system returns to an equilibrium state at the new temperature if δh is positive?
2 nh3(g) ⇄ n2(g) + 3 h2(g)
a. the concentration of ammonia, nh3, will increase.
b. the concentration of nitrogen gas, n2, will decrease.
c. the rate of the forward reaction will increase.
d. there will be no change; increasing the temperature will not change the position of equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
The reaction below is allowed to come to equilibrium. after equilibrium is reached, the temperature...
Questions


Social Studies, 21.10.2020 01:01






Spanish, 21.10.2020 01:01

History, 21.10.2020 01:01

Computers and Technology, 21.10.2020 01:01



Health, 21.10.2020 01:01



Mathematics, 21.10.2020 01:01


Health, 21.10.2020 01:01

Mathematics, 21.10.2020 01:01

Computers and Technology, 21.10.2020 01:01