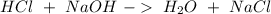The reactants of two chemical reactions are shown.
reaction 1: hcl and naoh
reaction 2:...

Chemistry, 01.07.2019 20:20 Nathaliasmiles
The reactants of two chemical reactions are shown.
reaction 1: hcl and naoh
reaction 2: cu and agno3
which reaction is likely to be a redox reaction?
a) reaction 1, because hydrogen and hydroxide atoms will be formed.
b) reaction 1, because all ions formed will retain their electrostatic charges.
c) reaction 2, because the electrostatic charge on copper ion will change.
d) reaction 2, because the charges on each side of the equation will be unequal.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
Questions


English, 16.12.2019 10:31

Mathematics, 16.12.2019 10:31

Mathematics, 16.12.2019 10:31

Mathematics, 16.12.2019 10:31

Geography, 16.12.2019 10:31


English, 16.12.2019 10:31

Mathematics, 16.12.2019 10:31

Mathematics, 16.12.2019 10:31




Chemistry, 16.12.2019 10:31


English, 16.12.2019 10:31

History, 16.12.2019 10:31