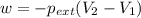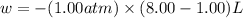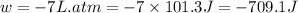Chemistry, 01.07.2019 18:20 jessica2138
One mole of an ideal gas is expanded from a volume of 1.00 liter to a volume of 8.00 liters against a constant external pressure of 1.00 atm. how much work (in joules) is performed on the surroundings? ignore significant figures for this problem. (t = 300 k; 1 l·atm = 101.3 j)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What are the concentrations of hydroxide and hydronium ions in a solution with a ph of 10.2?
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
One mole of an ideal gas is expanded from a volume of 1.00 liter to a volume of 8.00 liters against...
Questions

Mathematics, 13.11.2019 20:31

English, 13.11.2019 20:31

History, 13.11.2019 20:31

English, 13.11.2019 20:31



Chemistry, 13.11.2019 20:31


English, 13.11.2019 20:31

English, 13.11.2019 20:31


Mathematics, 13.11.2019 20:31


English, 13.11.2019 20:31

Mathematics, 13.11.2019 20:31


English, 13.11.2019 20:31

Mathematics, 13.11.2019 20:31


Social Studies, 13.11.2019 20:31

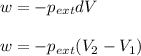
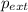 = external pressure = 1.00 atm
= external pressure = 1.00 atm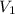 = initial volume of gas = 1.00 L
= initial volume of gas = 1.00 L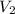 = final volume of gas = 8.00 L
= final volume of gas = 8.00 L