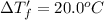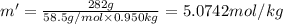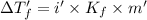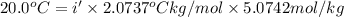Chemistry, 01.07.2019 16:10 whitneyb13
This problem has been solved! see the answerwhen 282. g of glycine (c2h5no2) are dissolved in 950. g of a certain mystery liquid x, the freezing point of the solution is 8.2c lower than the freezing point of pure x. on the other hand, when 282. g of sodium chloride are dissolved in the same mass of x, the freezing point of the solution is 20.0c lower than the freezing point of pure x. calculate the van't hoff factor for sodium chloride in x.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Consider three unlabeled bottles, each contain small pieces of one of the following metals. - magnesium - sodium - silver the following reagents are used for identifying the metals. - pure water - a solution of 1.0 molar hcl - a solution of concentrated hno3 (a) which metal can be easily identified because it is much softer than the other two? describe a chemical test that distinguishes this metal from the other two, using only one of the reagents above. write a balanced chemical equation for the reaction that occurs. (b) one of the other two metals reacts readily with the hcl solution. identify the metal and write the balanced chemical equation for the reaction that occurs when this metal is added to the hcl solution. use the table of standard reduction potentials (attached) to account for the fact that this metal reacts with hcl while the other does not. (c) the one remaining metal reacts with the concentrated hno3 solution. write a balanced chemical equation for the reaction that occurs. (d) the solution obtained in (c) is diluted and a few drops of 1 m hcl is added. describe what would be observed. write a balanced chemical equation for the reaction that occurs.
Answers: 2

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
This problem has been solved! see the answerwhen 282. g of glycine (c2h5no2) are dissolved in 950. g...
Questions




History, 03.03.2020 18:24

Mathematics, 03.03.2020 18:24

Mathematics, 03.03.2020 18:24

Mathematics, 03.03.2020 18:24





Physics, 03.03.2020 18:24





Physics, 03.03.2020 18:24


English, 03.03.2020 18:24

Mathematics, 03.03.2020 18:24

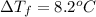
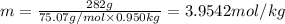
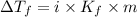
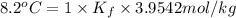
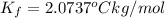 ..(1)
..(1)