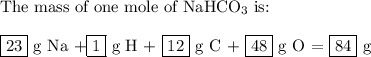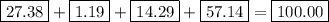Chemistry, 01.07.2019 00:10 lazerlemon500
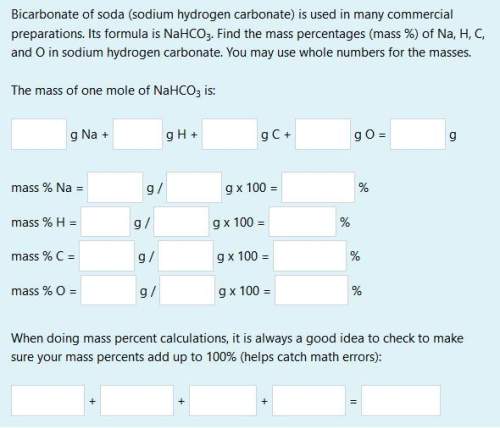
(need all boxes answered)
bicarbonate of soda (sodium hydrogen carbonate) is used in many commercial preparations. its formula is nahco3. find the mass percentages (mass %) of na, h, c, and o in sodium hydrogen carbonate. you may use whole numbers for the masses.
the mass of one mole of nahco3 is:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
(need all boxes answered)
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
bicarbonate of soda (sodium hydrogen carbonate) is used in many comm...
Questions

Mathematics, 13.04.2021 19:10

English, 13.04.2021 19:10


Mathematics, 13.04.2021 19:10


Mathematics, 13.04.2021 19:10




History, 13.04.2021 19:10


Mathematics, 13.04.2021 19:10





Physics, 13.04.2021 19:10

Social Studies, 13.04.2021 19:10

Physics, 13.04.2021 19:10