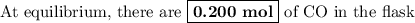Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
At a certain temperature, kc equals 1.4 × 102 for the reaction: 2 co(g) + o2(g) ⇌ 2 co2(g). if a 3....
Questions

Mathematics, 05.05.2020 08:37






Mathematics, 05.05.2020 08:37

Mathematics, 05.05.2020 08:37

Biology, 05.05.2020 08:37


English, 05.05.2020 08:37





Physics, 05.05.2020 08:37




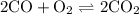
![\begin{aligned}\rm [O_{2}] &= \dfrac{0.100\;\rm mol}{3.50\;\rm L}\\\\&= 0.0285\;\rm mol/L\end{aligned}](/tpl/images/0029/8319/aeeca.png)
![\begin{aligned}\rm [CO_{2}] &= \dfrac{0.400\;\rm mol}{3.50\;\rm L}\\\\&= 0.1143\;\rm mol/L\end{aligned}](/tpl/images/0029/8319/147f6.png)
![\begin{aligned}\rm k_{eq} = \rm \dfrac{[CO_{2}]^{2}}{[CO]^{2}[O_{2}]} &= 1.4 \times 10^{2}\\\\\rm \dfrac{(0.1143)^{2}}{[CO]^{2}\times 0.0285} &= 1.4 \times 10^{2}\\\\\rm [CO]^{2}& = 0.00326\\\\&= 0.0571\end{aligned}](/tpl/images/0029/8319/58e18.png)

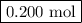
![\text{[O$_{2}$]} = \dfrac{\text{0.100 mol}}{\text{3.50 L}} = \text{0.02857 mol/L}\\\\\text{[CO$_{2}$]} = \dfrac{\text{0.400 mol}}{\text{3.50 L}} = \text{0.1143 mol/L}](/tpl/images/0029/8319/cf4c3.png)
![K_{\text{eq}} = \dfrac{\text{[CO$_{2}$]$^{2}$}}{\text{[CO]$^{2}$[O$_{2}$]}} = 1.4 \times10^{2}](/tpl/images/0029/8319/93a82.png)
![\begin{array}{rcl}\\\\\dfrac{0.1143^{2}}{\text{[CO]$^{2}$} \times 0.02857} & = & 1.4 \times10^{2}\\\\0.01306 & = & 4.000\text{[CO]$^{2}$}\\\\\text{[CO]$^{2}$} & = &\dfrac{0.01306}{4.000}\\\\\text{[CO]$^{2}$} & = & 0.003265\\\text{[CO]} & = & \mathbf{0.05714}\\\end{array}](/tpl/images/0029/8319/f60c0.png)