
Calculate the number of pounds of co2co2 released into the atmosphere when a 22.0 gallon22.0 gallon tank of gasoline is burned in an automobile engine. assume that gasoline is primarily octane, c8h18,c8h18, and that the density of gasoline is 0.692 g⋅ml−1.0.692 g⋅ml−1. this assumption ignores additives. also, assume complete combustion. co2co2 released:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Calculate the number of pounds of co2co2 released into the atmosphere when a 22.0 gallon22.0 gallon...
Questions

Mathematics, 26.05.2021 23:00


Mathematics, 26.05.2021 23:00

History, 26.05.2021 23:00

Mathematics, 26.05.2021 23:00

Mathematics, 26.05.2021 23:00


Chemistry, 26.05.2021 23:00


Spanish, 26.05.2021 23:00

Mathematics, 26.05.2021 23:00



Biology, 26.05.2021 23:00

Mathematics, 26.05.2021 23:00



Biology, 26.05.2021 23:00


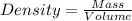
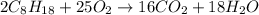
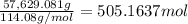
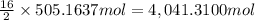 of carbon-dioxide
of carbon-dioxide

