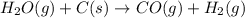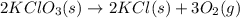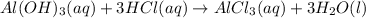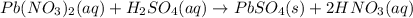Chemistry, 29.06.2019 01:20 ambernolinan
Indicate what type, or types, of reaction each of the following represents: (a) h2 o(g) + c(s) ⟶ co(g) + h2 (g) (b) 2kclo3 (s) ⟶ 2kcl(s) + 3o2 (g) (c) al(oh)3 (aq) + 3hcl(aq) ⟶ alcl3 (aq) + 3h2 o(l) (d) pb(no3 )2 (aq) + h2so4 (aq) ⟶ pbso4 (s) + 2hno3 (aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
Indicate what type, or types, of reaction each of the following represents: (a) h2 o(g) + c(s) ⟶ co...
Questions


Mathematics, 09.01.2021 07:50

Chemistry, 09.01.2021 07:50






English, 09.01.2021 07:50

Geography, 09.01.2021 07:50

Mathematics, 09.01.2021 07:50

Mathematics, 09.01.2021 07:50


Biology, 09.01.2021 07:50


Mathematics, 09.01.2021 07:50


Chemistry, 09.01.2021 07:50

Arts, 09.01.2021 07:50

Mathematics, 09.01.2021 07:50