Chemistry, 28.06.2019 23:30 springcoates
3. the rate law for the reaction nh4+(aq) + no2–(aq) → n2(g) + 2h2o(l) is given by rate = k[nh4+][no2–]. at 25ºc, the rate constant is 3.0 × 10–4/ m · s. calculate the rate of the reaction at this temperature if [nh4+] = 0.26 m and [no2–] = 0.080 m. (5 points)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
3. the rate law for the reaction nh4+(aq) + no2–(aq) → n2(g) + 2h2o(l) is given by rate = k[nh4+][no...
Questions



Mathematics, 15.04.2020 21:27


Social Studies, 15.04.2020 21:27




Mathematics, 15.04.2020 21:27


Mathematics, 15.04.2020 21:27


Chemistry, 15.04.2020 21:27

Chemistry, 15.04.2020 21:27

Mathematics, 15.04.2020 21:27


Mathematics, 15.04.2020 21:27



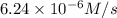 .
.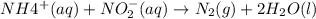
![[NH_4^{+}]=0.26 M](/tpl/images/0028/9019/f2f2d.png)
![[NO_2^{-}]=0.080 M](/tpl/images/0028/9019/214e5.png)
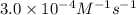
![R= k[NH_{4}^+][NO_{2}^-]](/tpl/images/0028/9019/cb361.png)