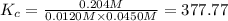Carbonyl chloride (cocl2), also called phosgene, was used in world war i as a poisonous gas. the equilibrium concentrations for the reaction between carbon monoxide and molecular chlorine to form carbonyl chloride at a certain temperature are [co] = 0.0210 m, [cl2] = 0.0450 m, and [cocl2] = 0.204 m. co(g) + cl2(g) ⇆ cocl2(g) calculate the equilibrium constant (kc).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
Carbonyl chloride (cocl2), also called phosgene, was used in world war i as a poisonous gas. the equ...
Questions

Mathematics, 13.04.2020 02:31

Mathematics, 13.04.2020 02:31

Mathematics, 13.04.2020 02:31



Social Studies, 13.04.2020 02:31

Chemistry, 13.04.2020 02:31


English, 13.04.2020 02:32

Biology, 13.04.2020 02:32



Mathematics, 13.04.2020 02:33

Mathematics, 13.04.2020 02:35

Mathematics, 13.04.2020 02:35

Mathematics, 13.04.2020 02:35




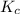
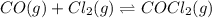
![[CO] = 0.0210 M](/tpl/images/0028/7944/23f4d.png)
![[Cl_2] = 0.0450 M](/tpl/images/0028/7944/558aa.png)
![[COCl_2] = 0.204 M](/tpl/images/0028/7944/1309b.png)
![K_c=\frac{[COCl_2]}{[CO][Cl_2]}](/tpl/images/0028/7944/36d91.png)