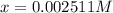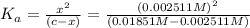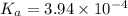Chemistry, 28.06.2019 21:30 tilievaughn14
Aspirin (acetylsalicylic acid, c9h8o4) is a weak monoprotic acid. to determine its acid-dissociation constant, a student dissolved 2.00 g of aspirin in 0.600 l of water and measured the ph. what was the ka value calculated by the student if the ph of the solution was 2.60?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1


Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Aspirin (acetylsalicylic acid, c9h8o4) is a weak monoprotic acid. to determine its acid-dissociation...
Questions

Mathematics, 14.12.2019 05:31

Chemistry, 14.12.2019 05:31

Business, 14.12.2019 05:31


English, 14.12.2019 05:31

Mathematics, 14.12.2019 05:31

Biology, 14.12.2019 05:31


Mathematics, 14.12.2019 05:31



English, 14.12.2019 05:31

Biology, 14.12.2019 05:31

Social Studies, 14.12.2019 05:31


Chemistry, 14.12.2019 05:31

Mathematics, 14.12.2019 05:31

English, 14.12.2019 05:31

Mathematics, 14.12.2019 05:31

Computers and Technology, 14.12.2019 05:31

 .
.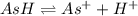
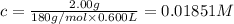
![K_a=\frac{[H^+][As^+]}{[AsH]}=\frac{x\times c}{(c-x)}=\frac{x^2}{(c-x)}](/tpl/images/0028/5803/345f7.png) ..(1)
..(1)![pH=2.60=\log[H^+]=-\log[x]](/tpl/images/0028/5803/39c79.png)