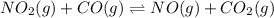Chemistry, 28.06.2019 21:20 tamikalove78
Consider the following reaction at equilibrium: no2(g) + co(g) = no(g) + co2(g) suppose the volume of the system is decreased at constant temperature, what change will this cause in the system? a shift to produce more no a shift to produce more co a shift to produce more no2 no shift will occur

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Consider the following reaction at equilibrium: no2(g) + co(g) = no(g) + co2(g) suppose the volume...
Questions


Biology, 17.09.2019 21:30



Mathematics, 17.09.2019 21:30


Mathematics, 17.09.2019 21:30





Mathematics, 17.09.2019 21:30

Biology, 17.09.2019 21:30


Social Studies, 17.09.2019 21:30



Mathematics, 17.09.2019 21:30


Business, 17.09.2019 21:30