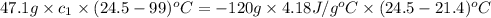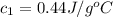Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
A47.1 g sample of a metal is heated to 99.0°c and then placed in a calorimeter containing 120.0 g of...
Questions



Mathematics, 06.05.2020 01:38

History, 06.05.2020 01:38

Computers and Technology, 06.05.2020 01:38

History, 06.05.2020 01:38



Mathematics, 06.05.2020 01:38

Mathematics, 06.05.2020 01:38

Mathematics, 06.05.2020 01:38

Mathematics, 06.05.2020 01:38




English, 06.05.2020 01:38



Mathematics, 06.05.2020 01:38

Mathematics, 06.05.2020 01:38

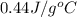 ).
).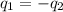
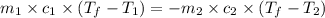
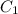 = specific heat of metal = ?
= specific heat of metal = ?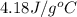
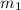 = mass of metal = 47.1 g
= mass of metal = 47.1 g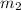 = mass of water = 120 g
= mass of water = 120 g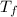 = final temperature of water =
= final temperature of water = 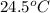
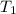 = initial temperature of metal =
= initial temperature of metal = 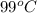
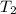 = initial temperature of water =
= initial temperature of water =