
Chemistry, 27.06.2019 20:30 oKINGDEROo
Which of the following compounds has polar covalent bonds: nabr, br2, hbr, and cbr4?
a) hbr and cbr4
b) br2 only
c) br2 and hbr
d) nabr only
e) hbr only

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
Which of the following compounds has polar covalent bonds: nabr, br2, hbr, and cbr4?
a) hbr...
a) hbr...
Questions

History, 11.10.2019 23:40


Social Studies, 11.10.2019 23:40



History, 11.10.2019 23:40


Mathematics, 11.10.2019 23:40


English, 11.10.2019 23:40


Mathematics, 11.10.2019 23:40

Biology, 11.10.2019 23:40



Biology, 11.10.2019 23:40



Chemistry, 11.10.2019 23:40

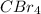 will be a covalent compound as there is sharing of electron between carbon and bromine atom.
will be a covalent compound as there is sharing of electron between carbon and bromine atom. 

