
Chemistry, 29.01.2020 19:02 Pizzapegasus1
Calculate the enthalpy change associated with the conversion of 25.0 grams of ice at -4.00 °c to water vapor at 110.0 °c. the specific heats of ice, water, and steam are 2.09 j/g-k, 4.18 j/g-k, and 1.84 j/g-k, respectively. for , δhfus = 6.01 kj/mol and δhvap = 40.67 kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Calculate the enthalpy change associated with the conversion of 25.0 grams of ice at -4.00 °c to wat...
Questions

Business, 29.08.2019 22:10



Mathematics, 29.08.2019 22:10

Health, 29.08.2019 22:10



Advanced Placement (AP), 29.08.2019 22:10



History, 29.08.2019 22:10

English, 29.08.2019 22:10

Mathematics, 29.08.2019 22:10


Mathematics, 29.08.2019 22:10

Mathematics, 29.08.2019 22:10


Mathematics, 29.08.2019 22:10


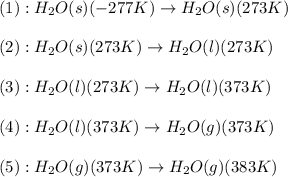
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0482/9975/e4ef0.png)
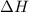 = enthalpy change = ?
= enthalpy change = ?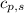 = specific heat of solid water = 2.09 J/gk
= specific heat of solid water = 2.09 J/gk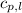 = specific heat of liquid water = 4.18 J/gk
= specific heat of liquid water = 4.18 J/gk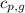 = specific heat of liquid water = 1.84 J/gk
= specific heat of liquid water = 1.84 J/gk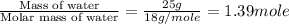
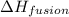 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole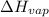 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[25g\times 4.18J/gK\times (273-277)k]+1.39mole\times 6010J/mole+[25g\times 2.09J/gK\times (373-273)k]+1.39mole\times 40670J/mole+[25g\times 1.84J/gK\times (383-373)k]](/tpl/images/0482/9975/b0606.png)
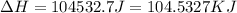 (1 KJ = 1000 J)
(1 KJ = 1000 J)

