Chemistry, 27.06.2019 10:10 Chatoloko231
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how many moles are present in this sample?
when answering the question, include the following:
state how to find the molar mass for the hydrocarbon.
state how you know if you need to multiply or divide by the molar mass.
give the correct number of significant figures and explain why the answer has that many significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how m...
Questions





Social Studies, 27.03.2020 05:16


Mathematics, 27.03.2020 05:16

Mathematics, 27.03.2020 05:16

Mathematics, 27.03.2020 05:16






Mathematics, 27.03.2020 05:16





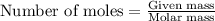
![[(2\times 12)+(6\times 1)]=30g/mol](/tpl/images/0022/9571/19171.png)