
Chemistry, 27.06.2019 03:20 xnadertheking
The value of δh° for the reaction below is -126 kj. the amount of heat that is released by the reaction of 10.0 g of na2o2 with water is kj. 2na2o2 (s) + 2h2o (l) → 4naoh (s) + o2 (g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
The value of δh° for the reaction below is -126 kj. the amount of heat that is released by the react...
Questions





Business, 02.12.2019 13:31



English, 02.12.2019 13:31


English, 02.12.2019 13:31


Mathematics, 02.12.2019 13:31

Mathematics, 02.12.2019 13:31


Health, 02.12.2019 13:31


Mathematics, 02.12.2019 13:31

History, 02.12.2019 13:31


 will be -8.064 kJ.
will be -8.064 kJ.
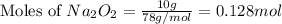

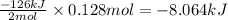 of energy.
of energy.

