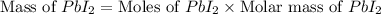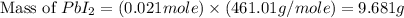The balanced equation for the reaction of aqueous pb(clo3)2 with aqueous nai is pb(clo3)2(aq)+2nai(aq)⟶pbi2(s)+2nac lo3(aq) what mass of precipitate will form if 1.50 l of concentrated pb(clo3)2 is mixed with 0.200 l of 0.210 m nai? assume the reaction goes to completion. mass of precipitate:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1


Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1
You know the right answer?
The balanced equation for the reaction of aqueous pb(clo3)2 with aqueous nai is pb(clo3)2(aq)+2nai(a...
Questions

Mathematics, 19.02.2021 01:00








Chemistry, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00


Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00




Mathematics, 19.02.2021 01:00

Physics, 19.02.2021 01:00


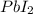 precipitate produced will be, 9.681 grams.
precipitate produced will be, 9.681 grams.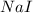 .
.
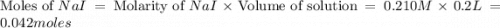
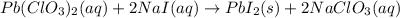
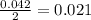 moles of
moles of