Chemistry, 05.02.2020 00:02 mercydiaz84
(a) write the balanced neutralization reaction that occurs between h2so4 and koh in aqueous solution. phases are optional. (b) suppose 0.750 l of 0.480 m h2so4 is mixed with 0.700 l of 0.290 m koh. what concentration of sulfuric acid remains after neutralization?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
(a) write the balanced neutralization reaction that occurs between h2so4 and koh in aqueous solution...
Questions




Mathematics, 24.03.2021 20:20

Arts, 24.03.2021 20:20

Mathematics, 24.03.2021 20:20

Spanish, 24.03.2021 20:20


Mathematics, 24.03.2021 20:20


Mathematics, 24.03.2021 20:20

Chemistry, 24.03.2021 20:20

Mathematics, 24.03.2021 20:20


Mathematics, 24.03.2021 20:20


Mathematics, 24.03.2021 20:20

Mathematics, 24.03.2021 20:20


Mathematics, 24.03.2021 20:20


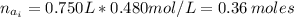
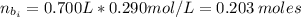
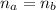
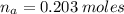
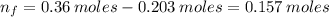
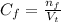
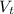 : is the total volume = (0.750 + 0.700) L = 1.45 L
: is the total volume = (0.750 + 0.700) L = 1.45 L