
Chemistry, 26.06.2019 05:20 sindy35111
The combustion of ethane (c2h6)(c2h6) produces carbon dioxide and steam. 2c2h6(g)+7o2(g)⟶4co2(g)+6h2o(g) 2c2h6(g)+7o2(g)⟶4co2(g)+6h2o(g) how many moles of co2co2 are produced when 5.95 mol5.95 mol of ethane is burned in an excess of oxygen? moles of co2: co2:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
The combustion of ethane (c2h6)(c2h6) produces carbon dioxide and steam. 2c2h6(g)+7o2(g)⟶4co2(g)+6h2...
Questions



Mathematics, 21.12.2021 22:30


Social Studies, 21.12.2021 22:30



Mathematics, 21.12.2021 22:30

World Languages, 21.12.2021 22:30

Physics, 21.12.2021 22:30






Chemistry, 21.12.2021 22:40

Mathematics, 21.12.2021 22:40



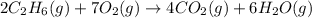
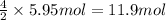 of carbon-dioxide
of carbon-dioxide

