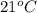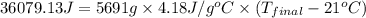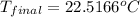Chemistry, 26.06.2019 05:10 kaitlan225
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 8.600 g c6h6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3


Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 8.600 g c...
Questions

Chemistry, 17.09.2019 03:00

History, 17.09.2019 03:00


Chemistry, 17.09.2019 03:00


Mathematics, 17.09.2019 03:00

History, 17.09.2019 03:00

History, 17.09.2019 03:00


English, 17.09.2019 03:00


Mathematics, 17.09.2019 03:00



Mathematics, 17.09.2019 03:00


Biology, 17.09.2019 03:00


Mathematics, 17.09.2019 03:00

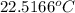
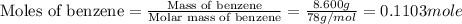
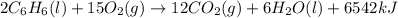
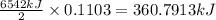 of energy on combustion.
of energy on combustion.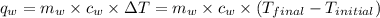
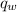 = heat released = 360.7913 kJ = 36079.13 J
= heat released = 360.7913 kJ = 36079.13 J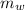 = mass of water = 5691 g
= mass of water = 5691 g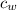 = specific heat of water=
= specific heat of water= 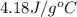
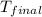 = final temperature = ?
= final temperature = ?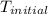 = initial temperature =
= initial temperature =