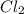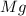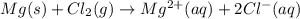Chemistry, 26.06.2019 04:20 tricklts15
Identify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent in the redox reaction. mg(s)+cl2(g)⟶mg2+(aq)+2cl−(aq) mg(s)+cl2(g)⟶mg2+(aq)+2cl−(aq) which substance gets oxidized? mgmg cl−cl− mg2+mg2+ cl2cl2 which substance gets reduced? cl2cl2 mg2+mg2+ mgmg cl−cl− what is the oxidizing agent? mg2+mg2+ cl−cl− mgmg cl2cl2 what is the reducing agent? cl2cl2 mgmg cl−cl− mg2+

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

You know the right answer?
Identify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent...
Questions


Mathematics, 12.05.2021 18:40

Mathematics, 12.05.2021 18:40

Mathematics, 12.05.2021 18:40


Mathematics, 12.05.2021 18:40

Mathematics, 12.05.2021 18:40

Mathematics, 12.05.2021 18:40



Mathematics, 12.05.2021 18:40



Mathematics, 12.05.2021 18:40


Biology, 12.05.2021 18:40

History, 12.05.2021 18:40

English, 12.05.2021 18:40

Mathematics, 12.05.2021 18:40

Mathematics, 12.05.2021 18:40