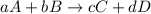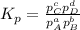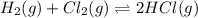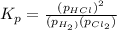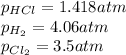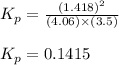Chemistry, 26.06.2019 03:20 elopezhilario6339
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibrium. the equilibrium pressure of hcl is found to be 1.418 atm. calculate kp for the reaction at this temperature. h2(g) + cl2(g) < => 2 hcl(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibri...
Questions

History, 19.11.2020 01:40

History, 19.11.2020 01:40

Mathematics, 19.11.2020 01:40


Mathematics, 19.11.2020 01:40







Chemistry, 19.11.2020 01:40

Business, 19.11.2020 01:40



Mathematics, 19.11.2020 01:40

Mathematics, 19.11.2020 01:40


Chemistry, 19.11.2020 01:40

Social Studies, 19.11.2020 01:40

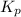 for the given chemical reaction is 0.1415
for the given chemical reaction is 0.1415