
Chemistry, 28.01.2020 01:31 JessTaylr04
Aself-contained underwater breathing apparatus (scuba) uses canisters containing potassium superoxide. the superoxide consumes the co2 exhaled by a person and replaces it with oxygen. 4 ko2(s) + 2 co2(g) n 2 k2co3(s) + 3 o2(g) what mass of ko2, in grams, is required to react with 8.90 l of co2 at 22.0 °c and 767 mm hg

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Aself-contained underwater breathing apparatus (scuba) uses canisters containing potassium superoxid...
Questions

Biology, 17.01.2021 22:30


Social Studies, 17.01.2021 22:30

Mathematics, 17.01.2021 22:30

Mathematics, 17.01.2021 22:30

Business, 17.01.2021 22:30



Biology, 17.01.2021 22:30





English, 17.01.2021 22:40

English, 17.01.2021 22:40





French, 17.01.2021 22:40

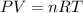
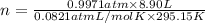
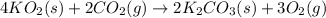
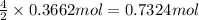 of potassium superoxide.
of potassium superoxide.

