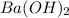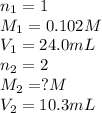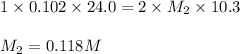Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
An aqueous solution of barium hydroxide is standardized by titration with a 0.102 m solution of perc...
Questions


Mathematics, 18.01.2022 04:00



Mathematics, 18.01.2022 04:00



Mathematics, 18.01.2022 04:00

Mathematics, 18.01.2022 04:00

Mathematics, 18.01.2022 04:00


Mathematics, 18.01.2022 04:00


History, 18.01.2022 04:00


Social Studies, 18.01.2022 04:00



Mathematics, 18.01.2022 04:00

Arts, 18.01.2022 04:00

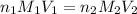
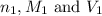 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 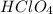
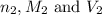 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is