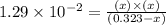Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
The equilibrium constant, kc, for the following reaction is 1.29×10-2 at 600 k. cocl2(g) co(g) + cl2...
Questions



Physics, 04.06.2020 13:01






Mathematics, 04.06.2020 13:01

Mathematics, 04.06.2020 13:01





Mathematics, 04.06.2020 13:01



Mathematics, 04.06.2020 13:01


Mathematics, 04.06.2020 13:01

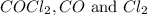 are, 0.2646, 0.0584 and 0.0584.
are, 0.2646, 0.0584 and 0.0584.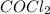 = 0.323 mole
= 0.323 mole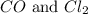 be, 'x'. So,
be, 'x'. So,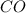 = x M
= x M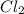 = x M
= x M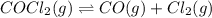
![K_c=\frac{[CO][Cl_2]}{[COCl_2]}](/tpl/images/0436/7314/59c52.png)