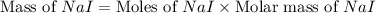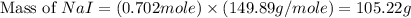Chemistry, 15.11.2019 14:31 ejfleck3655
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solution containing sodium iodide. 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) how many grams of sodium iodide, nai, nai, must be used to produce 89.1 g89.1 g of iodine, i2? i2? mass: g nai

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2
You know the right answer?
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solu...
Questions

Mathematics, 02.04.2020 01:27


Biology, 02.04.2020 01:27

Biology, 02.04.2020 01:27







Chemistry, 02.04.2020 01:27

Mathematics, 02.04.2020 01:27

Mathematics, 02.04.2020 01:27




Mathematics, 02.04.2020 01:27


English, 02.04.2020 01:27

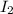 = 89.1 g
= 89.1 g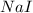 = 149.89 g/mole
= 149.89 g/mole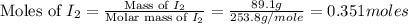
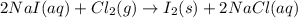
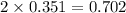 moles of
moles of