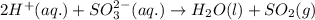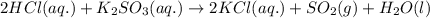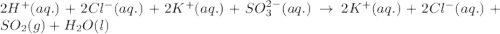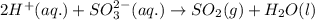Chemistry, 21.12.2019 17:31 basketball6076
Write a net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and potassium sulfite (aq) are combined. note: sulfites follow the same solubility trends as sulfates.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
Write a net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and potas...
Questions


Biology, 17.07.2019 06:00


Spanish, 17.07.2019 06:00

Mathematics, 17.07.2019 06:00





Mathematics, 17.07.2019 06:00

Mathematics, 17.07.2019 06:00

Mathematics, 17.07.2019 06:00

Mathematics, 17.07.2019 06:00


Mathematics, 17.07.2019 06:00

Biology, 17.07.2019 06:00



Spanish, 17.07.2019 06:00

Mathematics, 17.07.2019 06:00