
The explosive nitroglycerin (c3h5n3o9) decomposes rapidly upon ignition or sudden impact according to the following balanced equation: 4 c3h5n3o9 (l) → 12 co2 (g) + 10 h2o (g) + 6 n2 (g) + o2 (g) δrxnho = −5678 kj calculate the standard enthalpy of formation (δfho) for nitroglycerin. the enthalpy of formation of co2 (g) is -393.5 kj/mol. the enthalpy of formation of h2o (g) is -241.8 kj/mol.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
The explosive nitroglycerin (c3h5n3o9) decomposes rapidly upon ignition or sudden impact according t...
Questions



Computers and Technology, 28.01.2020 04:31








Mathematics, 28.01.2020 04:31









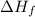 for
for 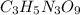 in the reaction is -365.5 kJ/mol.
in the reaction is -365.5 kJ/mol.![\Delta H_{rxn}=\sum [n\times \Delta H_f_{(product)}]-\sum [n\times \Delta H_f_{(reactant)}]](/tpl/images/0445/1737/18a63.png)
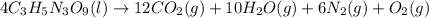
![\Delta H_{rxn}=[(12\times \Delta H_f_{(CO_2)})+(10\times \Delta H_f_{(H_2O)})+(6\times \Delta H_f_{(N_2)})+(1\times \Delta H_f_{(O_2)})]-[(4\times \Delta H_f_{(C_3H_5N_3O_9)})]](/tpl/images/0445/1737/c0f93.png)
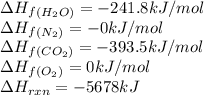
![-5678=[(12\times (-393.5))+(10\times (-241.8))+(6\times (0))+(1\times (0))]-[(4\times \Delta H_f_{(C_3H_5N_3O_9)})]\\\\\Delta H_f_{(C_3H_5N_3O_9)}=-365.5kJ/mol](/tpl/images/0445/1737/dcb72.png)


