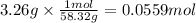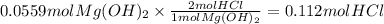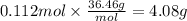Chemistry, 16.10.2019 23:30 wiljoystoltz253
Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily hcl, according to the reaction mg(oh)2(aq)+2hcl(aq)→2h2o(l)+mgcl2( aq) what mass of hcl, in grams, is neutralized by a dose of milk of magnesia containing 3.26 g of mg(oh)2? express the mass in grams to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

You know the right answer?
Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily...
Questions





English, 16.11.2020 17:30

Mathematics, 16.11.2020 17:30



Business, 16.11.2020 17:30







Chemistry, 16.11.2020 17:30


Business, 16.11.2020 17:30