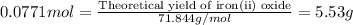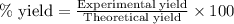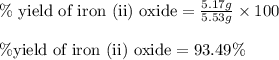Chemistry, 26.10.2019 11:43 Kathryn014
For the following reaction, 4.31 grams of iron are mixed with excess oxygen gas . the reaction yields 5.17 grams of iron(ii) oxide . iron ( s ) + oxygen ( g ) iron(ii) oxide ( s ) what is the theoretical yield of iron(ii) oxide ? 21.6 grams what is the percent yield for this reaction ? 85 %

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1


Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1
You know the right answer?
For the following reaction, 4.31 grams of iron are mixed with excess oxygen gas . the reaction yield...
Questions




Social Studies, 26.05.2021 06:30

Mathematics, 26.05.2021 06:30




Mathematics, 26.05.2021 06:30




Business, 26.05.2021 06:30

Biology, 26.05.2021 06:30


Mathematics, 26.05.2021 06:30



Mathematics, 26.05.2021 06:30

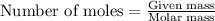 ....(1)
....(1)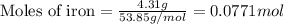
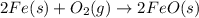
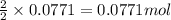 of iron (ii) oxide
of iron (ii) oxide