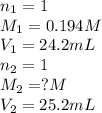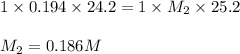An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

You know the right answer?
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of h...
Questions


Mathematics, 03.11.2019 05:31


Mathematics, 03.11.2019 05:31

English, 03.11.2019 05:31

Arts, 03.11.2019 05:31

Mathematics, 03.11.2019 05:31

Social Studies, 03.11.2019 05:31

English, 03.11.2019 05:31




History, 03.11.2019 06:31

Mathematics, 03.11.2019 06:31

Mathematics, 03.11.2019 06:31



Business, 03.11.2019 06:31


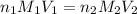
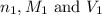 are the n-factor, molarity and volume of acid which is HBr
are the n-factor, molarity and volume of acid which is HBr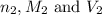 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.