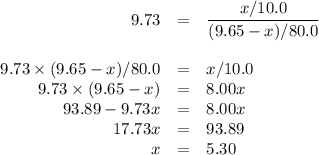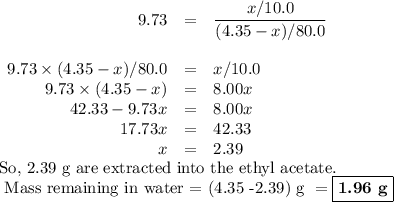Chemistry, 30.01.2020 11:45 andreimru123123
Assume an organic compound has a partition coefficient between water and ethyl acetate equal to 9.73. if there are initially 9.65 grams of the compound dissolved in 80.0 ml of water, how many grams will remain in the aqueous layer after extraction with two 10.0 ml portions of ethyl acetate?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Assume an organic compound has a partition coefficient between water and ethyl acetate equal to 9.73...
Questions

Mathematics, 10.11.2020 17:40



Chemistry, 10.11.2020 17:40

Business, 10.11.2020 17:40


Social Studies, 10.11.2020 17:40


Chemistry, 10.11.2020 17:40



Spanish, 10.11.2020 17:40

History, 10.11.2020 17:40


English, 10.11.2020 17:40




Spanish, 10.11.2020 17:50

Arts, 10.11.2020 17:50