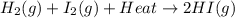The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

You know the right answer?
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this r...
Questions



Mathematics, 23.02.2021 21:50

Mathematics, 23.02.2021 21:50

Chemistry, 23.02.2021 21:50

Mathematics, 23.02.2021 21:50


Biology, 23.02.2021 21:50


Mathematics, 23.02.2021 21:50




Mathematics, 23.02.2021 21:50


Geography, 23.02.2021 21:50


Mathematics, 23.02.2021 21:50