Chemistry, 24.06.2019 08:00 nancyrj3860
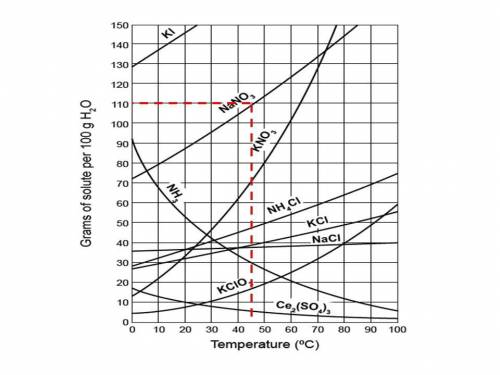
How many grams of nano3 would have to be added to 100. grams of water at 45°c to make a saturated solution of this salt? 1. 100. 2. 110. 3. 120. 4. 130.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
How many grams of nano3 would have to be added to 100. grams of water at 45°c to make a saturated so...
Questions

Social Studies, 04.03.2021 21:50

History, 04.03.2021 21:50

Mathematics, 04.03.2021 21:50


Mathematics, 04.03.2021 21:50


Mathematics, 04.03.2021 21:50

Mathematics, 04.03.2021 21:50

Mathematics, 04.03.2021 21:50

Physics, 04.03.2021 21:50


English, 04.03.2021 21:50



English, 04.03.2021 21:50

Mathematics, 04.03.2021 21:50



Mathematics, 04.03.2021 21:50