
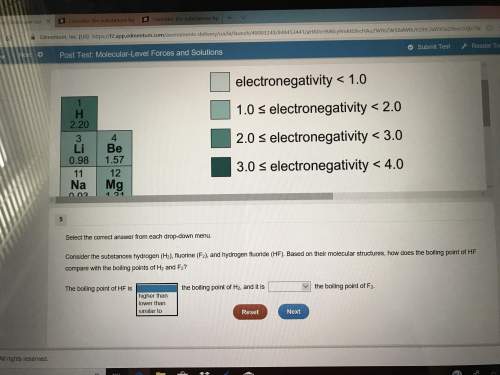
Plz don’t have much time left(picture included)select the correct answer from each drop-down menu. consider the substances hydrogen (h2), fluorine (f2), and hydrogen fluoride (hf). based on their molecular structures, how does the boiling point of hf compare with the boiling points of h2 and f2? the boiling point of hf is the boiling point of h2, and it is the boiling point of f2second set of opinions is the same

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

You know the right answer?
Plz don’t have much time left(picture included)select the correct answer from each drop-down menu....
Questions


History, 20.08.2020 06:01

Health, 20.08.2020 06:01

Mathematics, 20.08.2020 06:01



Mathematics, 20.08.2020 06:01


Mathematics, 20.08.2020 06:01


Mathematics, 20.08.2020 06:01


Mathematics, 20.08.2020 06:01




English, 20.08.2020 06:01

History, 20.08.2020 06:01

Chemistry, 20.08.2020 06:01




