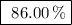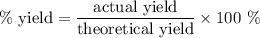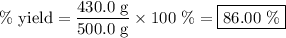Chemistry, 25.06.2019 08:00 saadizak7098
Achemical company produces ammonia using the following reaction: n2 + 3h2 → 2nh3they run a reaction meant to fill an order for a customer who would like to purchase 500.0g of ammonia. when the reaction is complete, the company finds they produced only 430.0g of ammonia. what it their percent yield for that reaction? 70.00%86.00%116.3%93.00%

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Achemical company produces ammonia using the following reaction: n2 + 3h2 → 2nh3they run a reaction...
Questions

History, 24.09.2020 16:01




History, 24.09.2020 16:01

Biology, 24.09.2020 16:01






Mathematics, 24.09.2020 16:01

Mathematics, 24.09.2020 16:01

Biology, 24.09.2020 16:01


Chemistry, 24.09.2020 16:01

Mathematics, 24.09.2020 16:01

English, 24.09.2020 16:01