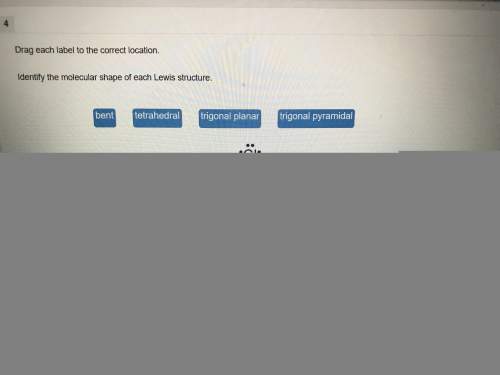
Chemistry, 25.06.2019 19:50 summerdooleyu
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is produced. b. co2 concentration increases. c. the equilibrium is pushed in the direction of reactants. d. nothing


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
What is the effect of adding more water to the following equilibrium reaction? a. more h2co3 is pro...
Questions







Physics, 18.12.2020 08:50

Mathematics, 18.12.2020 08:50


Mathematics, 18.12.2020 08:50

History, 18.12.2020 08:50

History, 18.12.2020 08:50



Mathematics, 18.12.2020 09:00

Biology, 18.12.2020 09:00



English, 18.12.2020 09:00