
Chemistry, 25.01.2020 00:31 brittanysanders
How much heat is released when 105g of steam at 100.0c is cooled to ice at -15c? enthalpy of vaporization of water is 40.67kj/mol, the enthalpy of fusion of water is 6.01kj/mol, the molar heat capacity of water is 75.4j/(mol. c) and the molar heat capacity of ice is 36.4j/(mol. c)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
How much heat is released when 105g of steam at 100.0c is cooled to ice at -15c? enthalpy of vapori...
Questions

World Languages, 14.06.2021 08:50

Chemistry, 14.06.2021 08:50

English, 14.06.2021 08:50



Mathematics, 14.06.2021 08:50

English, 14.06.2021 08:50

Advanced Placement (AP), 14.06.2021 08:50


Mathematics, 14.06.2021 08:50

Mathematics, 14.06.2021 08:50


Biology, 14.06.2021 09:00




Physics, 14.06.2021 09:00

Engineering, 14.06.2021 09:00


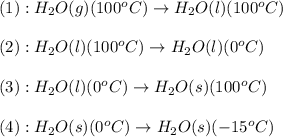
![\Delta H=n\times \Delta H_{condensation}+[n\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{freezing}+[n\times c_{p,s}\times (T_{final}-T_{initial})]](/tpl/images/0469/7239/e91c9.png)
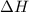 = enthalpy change = ?
= enthalpy change = ?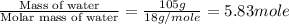
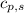 = specific heat of solid water =
= specific heat of solid water = 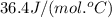
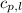 = specific heat of liquid water =
= specific heat of liquid water = 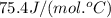
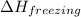 = enthalpy change for freezing = enthalpy change for fusion = - 6.01 KJ/mole = - 6010 J/mole
= enthalpy change for freezing = enthalpy change for fusion = - 6.01 KJ/mole = - 6010 J/mole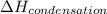 = enthalpy change for condensation = enthalpy change for vaporization = -40.67 KJ/mole = -40670 J/mole
= enthalpy change for condensation = enthalpy change for vaporization = -40.67 KJ/mole = -40670 J/mole![\Delta H=5.83mole\times -40670J/mole+[5.83mole\times 75.4J/(mol.^oC)\times (0-100)^oC]+5.83mole\times -6010J/mole+[5.83mole\times 36.4J/(mol.^oC)\times (-15-0)^oC]](/tpl/images/0469/7239/b7d7d.png)
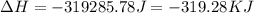 (1 KJ = 1000 J)
(1 KJ = 1000 J)


